Bubbling ship exhaust into the ocean
When the obvious questions give stupid answers, it’s time to ask stupid questions

Consider a fairly typical CO2 molecule that leaves a tailpipe, spends 500 years in the air absorbing IR photons and warming the earth, and then dissolves into the ocean more-or-less permanently[1]. Based on almost any proposed social cost of carbon, most of the harm from that emitted CO2 happens when it’s in the atmosphere, and much less when it’s in the ocean. So in situations where we must emit CO2, would it not be less harmful to short-cut the atmosphere part and emit the CO2 directly into the ocean, rather than into the air?
Say you wanted to decarbonize global shipping. That would mean redesigning big boats like Suezmax cargo ships, 200 million kg monsters with 30MW engines that can go weeks at a time on the very cheapest fuels, fossil sludge so thick it’s almost asphalt.
The obvious question is whether we can electrify the ships with batteries, or perhaps power them with alternative fuels. Smart people disagree but electrifying a Suezmax ship might take 60 million kg of Li-ion batteries[2], multiplying its cost and cutting its cargo capacity by almost half. That’s a hard sell. And the other straightforward decarbonization options – e-methanol, ammonia, biofuels —are to varying degrees even worse. That’s why there’s been hardly any progress in decarbonizing heavy marine transport.
When the obvious questions give stupid answers, it’s time to ask stupid questions. Heavy fuel oil is cheap and modern two-stroke marine diesels are amazingly economical to run. Much of that carbon is going to end up in the ocean anyway, and for a ship the ocean is right there, ready to absorb the CO2 emissions before they do too much harm. So what happens if you just bubble ship exhaust into the ocean?
Would the CO2 dissolve into the ocean at all?
[TLDR for this section: yes, easily and completely]
A below-waterline exhaust system would probably be combined with bubble-hull lubrication, and those systems typically work with millimeter-scale bubbles, so for these estimates we consider bubbles of radius 1 mm. We assume that pressure, temperature, and volume are constant and that the water is pure. For the pressure, we will find that most of the CO2 dissolves in the first meter of the bubble’s motion, which corresponds to a pressure change of no more than 10%. The bubble thermally equilibrates with the ocean on a timescale of ~0.1 s, so we can treat the temperature as constant. Then only mass transfer would change the volume, but ship exhaust is 5% CO2 by volume. Most of the remainder is insoluble nitrogen, so CO2 dissolution will not reduce the volume significantly. Finally, the marine mixed layer is in equilibrium with 400 ppm CO2, so it is heavily undersaturated relative to 5% CO2. We can thus approximate it as pure water.
Using empirical estimates of drag coefficients, we find a typical vertical terminal velocity of 0.2 m/s in still water. We ignore the flow induced by the ship’s motion, and assume that all bubbles rise at 0.2 m/s.
Because the bubble surface area remains approximately constant, the rate of dissolution is proportional to the concentration of CO2 remaining in the bubble. The concentration thus decays exponentially; we estimate its half-life. By Fick’s law, the rate of dissolution is proportional to the normal derivative of the aqueous CO2 concentration at the bubble’s surface. This gradient is enhanced by the bubble’s velocity, as the moving fluid sweeps dissolved CO2 away from the bubble.
The nature of the background fluid flow has a large effect on the rate of mass transfer. Large bubbles maintain free boundaries with large fluid velocities parallel to the surface. This advection greatly enhances mass transfer. However, small bubbles quickly become contaminated by surfactants that rigidify the surface. This leads to a no-slip fluid boundary condition, which damps the advective enhancement of dissolution. Empirically, the transition from small to large occurs at the millimeter scale, so our bubbles lie in a complex intermediate mass transfer regime.
We draw on theory and experiment for rigid and free bubbles to estimate the rate of mass transfer. We find a half-life of about 1s for the CO2 concentration. This estimate is within 40% of various experimental results. In particular, Olsen et al. (2017) study small (r < 400 µm) CO2 bubbles in seawater, and remark that “due to the high solubility of CO2, the bubbles are depleted of CO2 within seconds.”
During the 1s half-life, the bubbles rise 0.2 m. We refer to this as the “half-distance.” We plot this distance over a wider range of bubble size:

Approximate half-distance for millimeter-scale bubbles.
At this scale, the half-distance is roughly 100 radii. The curve is non-monotone due to the transition from rigid to free bubbles.
According to this estimate, once the bubbles have risen 1 m, they have lost > 95% of their CO2. This justifies our assumption of constant pressure, as the relevant part of the process occurs in a narrow pressure range. Moreover, a ship would inject bubbles at least several meters below the waterline (typical drafts are 10-15m for medium to large cargo vessels). Therefore, our calculations suggest that nearly all the exhaust CO2 will enter solution before it reaches the ocean surface. This conclusion is robust to moderate (order one) changes to the assumptions above.
Throughout, we have assumed that the background concentration of CO2 in seawater is zero. This would break down if the exhaust saturated the water surrounding the ship. As a test case, we consider a 350 m long 100k DWT container ship loaded to 60k tons steaming at 16 kn. At a typical engine load of 23 MW, it produces about 50 m3/s (55 kg/s) of exhaust. The dimensionless constant in Henry’s law for CO2 at STP is close to 1, so the ship exhaust would saturate about 20 m3/s of seawater. On the other hand, the ship displaces about 1400 m3/s of seawater through its forward motion. Thus, if the exhaust perfectly mixed in the displaced volume, it would be 30x undersaturated. This would not significantly slow dissolution. Perfect mixing seems unlikely, but 5% mixing would suffice.
How long would the CO2 stay in the ocean?
[TLDR for this section: in most cases less than 10% of the CO2 would still be in the ocean after 100 years]
To estimate how long the dissolved CO2 from the ship will stay in the ocean, we added CO2 to the top layer of the UVic Earth System Climate model and compared its evolution with an unperturbed model run. This is roughly equivalent to increasing the CO2 concentration uniformly in the first 50 m of the global ocean. After spatial averaging, the results agree well with a one-dimensional diffusion model on the half-line with zero boundary data (signifying the unperturbed atmospheric concentration).

Blue —total CO2 that has outgassed to the atmosphere in the UVic model.
Gold — exponential fit. Green — 1D diffusion fit.
Because the UVic model is nearly linear, these results closely approximate the geographical mean of outgassing. Only about 5% of the CO2 remains in the ocean after 100 years[3]. This is no surprise—we are injecting CO2 into the mixed layer of the ocean, which is nearly in equilibrium with the atmosphere. Only a small fraction of the CO2 remains isolated from the atmosphere after decades. This fraction likely corresponds to carbon that has diffused downward across the thermocline.
The mean sequestration rate is low, but we expect some areas of the ocean to retain much more carbon due to downwelling. To test this hypothesis, we examine the spatial distribution of the pulse carbon that has moved below 200 m in the early stage of the UVic model run.
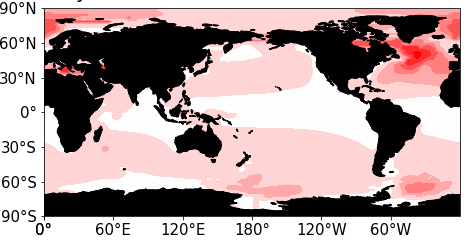
Concentration of excess CO2 below 200 m depth in UVic model 20 years after pulse. This approach to CO2 persistence came from Ken Caldeira, and the simulations were conducted with Long Cao.
These results reflect the strong downwelling in the subpolar North Atlantic. Given the high degree of heterogeneity, it seems reasonable to conjecture that the North Atlantic sequesters carbon several times better than average. If this is true, 10–20% of North Atlantic ship exhaust could remain sequestered for 100 years[4].
The North Atlantic hosts about 10% of global shipping, so a worldwide implementation of the ship-bubbling strategy might sequester 5-7% of global shipping emissions. Since global shipping emits ~1 GtCO2/yr, that makes bubbling ship exhaust into the ocean a <100 MtCO2/yr strategy.
But it might be cheap
The idea of a retrofit to discharge engine exhaust underwater sounds like it’d be pure hassle to a shipbuilder, with no economic rationale. But there are two reasons it might be a net moneymaker:
Sulfur scrubbing
In 2020, the International Maritime Organization tightened restrictions on sulfur content in ship exhaust. Setting aside for now the question of whether desulfurization is indeed beneficial for the environment[5], it set off a scramble among ship owners: to either begin purchasing low-sulfur (0.5% rather than ~3%) fuel, which increases fuel costs by as much as 40%, or else to install sulfur-scrubbing systems, which cost about $2-4M.
Some scrubbers are “open loop”—spraying seawater into the exhaust stream to capture sulfur dioxide in solution, and continuously dumping the water overboard. “Closed loop” variants sometimes include a consumable base to increase the concentration of the discharge solution. However, in both cases, all of the sulfur is eventually discharged to the ocean as an acidic solution.
Based on the 2 order of mag higher solubility of SO2 than CO2 in seawater (~100g/L vs ~2.5g/L at 10ºC), we expect that a below-waterline exhaust system would meet both the air-release and water discharge standards set forth by the IMO without employing any separate sulfur scrubbing system.
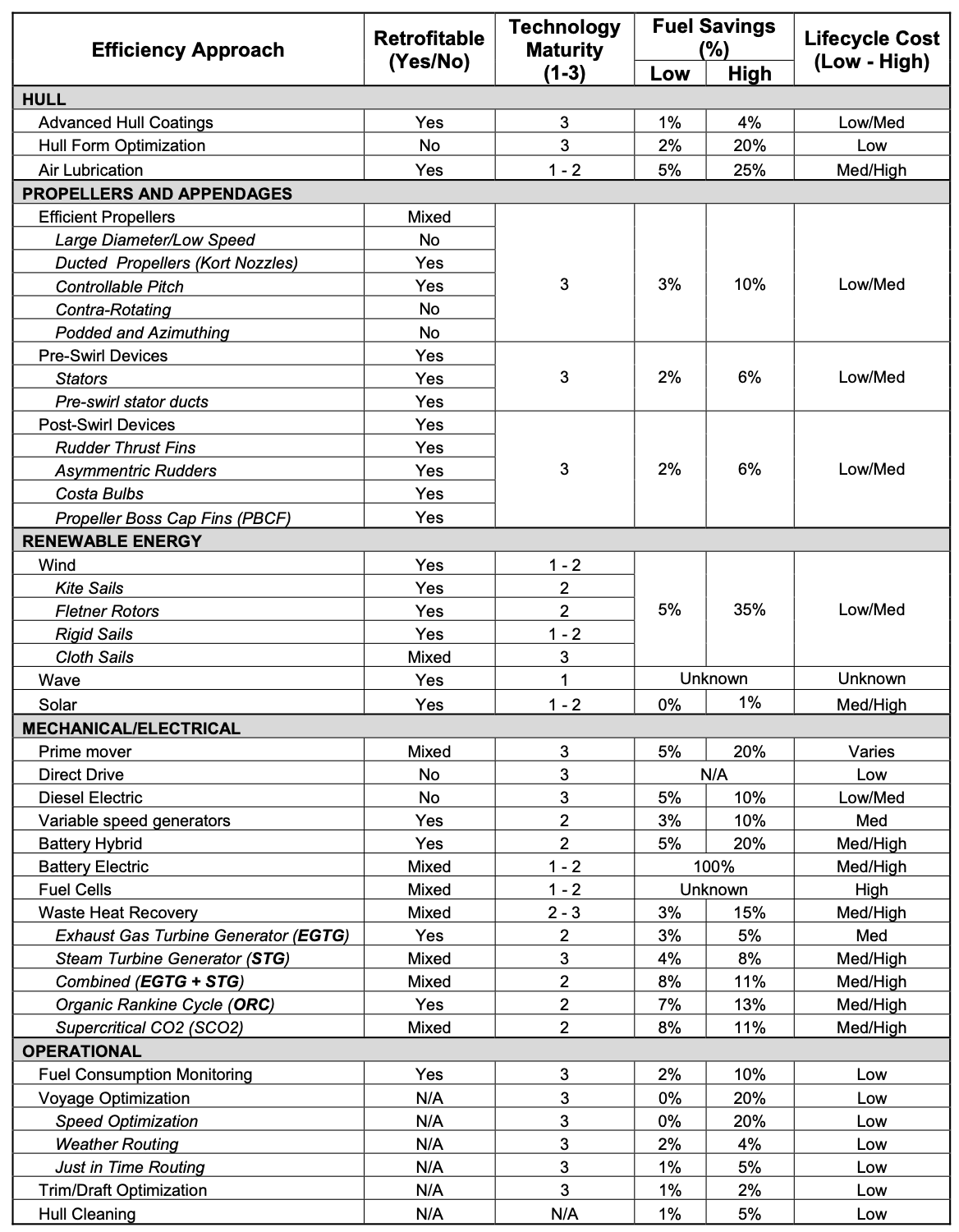
Air lubrication
Bubbles released underneath a ship hull can substantially decrease hydrodynamic drag, by effectively decreasing liquid density and viscosity. So-called “air lubrication systems” are a fairly mature technology that generally increase fuel efficiency from 5-20%. Air lubrication systems are standard on new Royal Caribbean vessels, and have been installed as retrofits and as built-in components of Panamax and Suezmax vessels.
One cool technical aspect of ship air lubrication systems is that no specialty nozzles or other gizmos are required to create the carpet of 1-3mm bubbles. Rather, air is pumped into passive cavities in the ship hull, from which a Kelvin-Helmholtz instability at the liquid-air boundary creates the bubble-water mixture.

The obvious thing to do is plumb the ship's engine exhaust into air lubrication systems that are already used improve hydrodynamics on cargo ships. Silverstream air lubrication retrofit (left). Mitsubishi air lubrication system (right). Source: Fotopolous et al.
The amount of air typically used for lubrication is at the low end of usual exhaust flow rates for a ship of a given size. In one study of the Foreship air lubrication system on a cruise ship, 10kg/s of air were provided at 0.9 bar pressure, for a 7% fuel savings at an engine power of 20MW. At 20MW power and a typical specific fuel consumption of 0.16 kg/kWhr, the exhaust flow rate was ~50kg/s—on the same order but higher than the typical demand for lubricating air. For other ship hull-engine combinations (notably cruise ships, which burn a lot of fuel in order to generate electricity) more exhaust is generated than would be required for air lubrication systems. We haven’t seen investigations of air lubrication in higher air-flow regimes; if it turned out to be unhelpful or uneconomical to increase the amount of lubricating air to match the exhaust flow rate, then you’d be faced with only mixing a portion of the exhaust into the ocean; if this fraction fell below about 80%, you’d lose the ability to use full-sulfur fuel without an additional scrubbing system.
You might spitball that using ship exhaust in an air lubrication system might cost a little less than installing separate air lubrication and sulfur scrubbing systems[6]. That means that as near as we can tell, bubbling exhaust into the ocean might pay for itself on air lubrication and sulfur scrubbing benefits, meaning the CO2 reduction comes for free.
Still, nobody is going to do this
So bubbling ship exhaust into the ocean might be a way to eliminate 5-7% of cargo ship emissions at near zero cost. Combine the new exhaust system for bubble-carpet lubrication of the ship’s hull and the thing could even be a moneymaker. But we don’t think anybody is going to do it.
First, if the shipping industry had the gumption for this type of complex re-engineering of their ships for the sake of emissions reduction, there is a lot of lower-hanging fruit they’d already be tackling. The biggest one is sailing slower. Bulk carriers (as opposed to container ships) are the vast majority of total ocean freight and they don’t need to move fast; if electrification and automation can reduce crew needs, then massive energy savings could offset the increased crew and capital costs of going slower (half the speed takes a quarter the energy but costs double for ship and crew). Even without going slower, there are a lot of efficiency improvements that have been developed (amazing list page 102)[7] that could easily add up to a 25% decrease in fuel consumption without major cost or speed changes—effective but unsexy stuff like optimizing itineraries, improving propellers or hull design, measuring fuel flow better etc.
But the shipping industry isn’t doing these things. Like with so many old global industries, shipping companies operate like money printers. People employed on the money printer are disincentivized from throwing any wrenches in it. Shippers probably accept poorly optimized ships to reduce schedule risk; shipyards are probably willing to give you a fixed price bid on ship design they’ve built hundreds of times, but one with efficiency features probably comes with all sorts of contract caveats. This means a clean-sheet electric or hybrid-electric design probably isn’t quite as unlikely as the basic math might make it seem, since it will be able to incorporate lots of efficiency-boosting design features that conventional ships have been slow to adopt. But it leaves a complicated but incremental improvement like a combined air lubrication / sulfur scrubbing / exhaust bubbler system a hard sell.
We also doubt that the world would view the “ship bubbles” strategy too favorably even if someone is willing to build it. People can be weird about trading one harm for another[8]. Ocean acidification is a harm that you’d be guilty of explicitly[9], while CO2 emissions are priced in as a pre-existing global problem that you can’t meaningfully be singled out for, since everyone’s already complicit to some degree. People might see you as the bad guy for mainlining kilotons of toxic exhaust into the ocean, even if you could wave pages of math at them proving that it was going to wind up there anyway, and that you were reducing net harm versus releasing it to the atmosphere.
Overall, we’re looking for startup opportunities and this clearly isn’t one. Since 5-7% is a low overall sequestration fraction and suffers a built-in PR problem, we doubt that there would be any takers for this strategy. An additional problem is that the gambit offers many marginal value centers (sulfur scrubbing, air lubrication, CO2 mitigation), but no single clearly winning aspect that could serve as lynchpin of a go-to-market strategy. Not worth pursuing :(.
References
[1] Vaclav Smil, Prime Movers of Globalization, MIT Press, 2010.
[2] J. E. Olsen, D. Dunnebier, E. Davies, P. Skjetne, and J. Morud, “Mass transfer between bubbles and seawater,” Chem. Eng. Sci., vol. 161, pp. 308–315, Apr. 2017, doi: 10.1016/j.ces.2016.12.047.
[3] A. Tomiyama, I. Kataoka, I. Zun, and T. Sakaguchi, “Drag Coefficients of Single Bubbles under Normal and Micro Gravity Conditions,” JSME Int. J. Ser. B, vol. 41, no. 2, pp. 472–479, 1998, doi: 10.1299/jsmeb.41.472.
[4] A. I. Johnson, F. Besik, and A. E. Hamielec, “Mass transfer from a single rising bubble,” Can. J. Chem. Eng., vol. 47, no. 6, pp. 559–564, 1969, doi: 10.1002/cjce.5450470615.
[5] G. A. Hughmark, “Liquid-Liquid Spray Column Drop Size, Holdup, and Continuous Phase Mass Transfer,” Ind. Eng. Chem. Fundam., vol. 6, no. 3, pp. 408–413, Aug. 1967, doi: 10.1021/i160023a014.
[6] F. H. Garner and R. D. Suckling, “Mass transfer from a soluble solid sphere,” AIChE J., vol. 4, no. 1, pp. 114–124, 1958, doi: 10.1002/aic.690040120.
[7] Higbie, R, “The rate of absorption of a pure gas into a still liquid during short periods of exposure,” Trans AIChE, vol. 31, pp. 365–389, 1935.
[8] F. Takemura and A. Yabe, “Rising speed and dissolution rate of a carbon dioxide bubble in slightly contaminated water,” J. Fluid Mech., vol. 378, pp. 319–334, Jan. 1999, doi: 10.1017/S0022112098003358.
[9] F. Deindoerfer and A. Humphrey, “Mass Transfer from Individual Gas Bubbles,” Ind. Eng. Chem., vol. 53, no. 9, pp. 755–759, Sep. 1961, doi: 10.1021/ie50621a035.
[10] International Maritime Organization, 2010 Guidelines for Exhaust Gas Cleaning Systems, MEPC.259(68), Section 10.1, 2015.
[11] J. Moldanová, et al., “Exhaust gas scrubbing in marine engines: Influence on emissions and water discharges,” J. Mar. Sci. Eng., vol. 8, no. 4, p. 299, 2020, doi: 10.3390/jmse8040299.
[12] B. Starcrest Consulting Group, Evaluation of the Water Discharge from Exhaust Gas Cleaning Systems on Vessels Operating in California Waters, California Air Resources Board, Apr. 2021. [Online]. Available: https://escholarship.org/content/qt13t3t231/qt13t3t231.pdf
[13] O. J. Nielsen, M. S. Johnson, and H. B. Karlsson, “Atmospheric chemistry of sulfur compounds in ship plumes,” Atmos. Chem. Phys., vol. 8, no. 9, pp. 2387–2395, 2008, doi: 10.5194/acp-8-2387-2008.
[14] MAN Energy Solutions, Project Guide – Four-Stroke Marine Engines, L2832H Series, MAN ES Publication, 2022. [Online]. Available: https://man-es.com/applications/projectguides/4stroke/manualcontent/Mobile/PG_M-III_L2832H.pdf
[15] P. K. Patankar and S. T. Siingh, “Effect of aerosols on cloud formation over marine environments,” J. Atmos. Sci., vol. 57, no. 16, pp. 2684–2699, 2000. [Online]. Available: https://journals.ametsoc.org/view/journals/atsc/57/16/1520-0469_2000_057_2684_eoaoca_2.0.co_2.xml
[16] C. Paris, “Maritime emissions rule triggers split in shipping costs,” The Wall Street Journal, Dec. 23, 2019. [Online]. Available: https://www.wsj.com/articles/maritime-emissions-rule-triggers-split-in-shipping-costs-11576839601.
[17] Bryan Comer and Dan Rutherford, “Air and water pollution from ships with scrubbers,” The International Council on Clean Transportation (ICCT), Jan. 2020. [Online]. Available: https://theicct.org/publications/air-water-pollution-scrubbers-2020.
[18] A. I. Partanen, A. Laakso, A. Schmidt, H. Kokkola, T. Kuokkanen, J.-P. Pietikäinen, V.-M. Kerminen, K. E. J. Lehtinen, L. Laakso, and H. Korhonen, “Climate and air quality trade-offs in altering ship fuel sulfur content,” Atmos. Chem. Phys., vol. 13, no. 23, pp. 12059–12071, 2013, doi:10.5194/acp-13-12059-2013.
[19] T. Yuan, H. Song, R. Wood, C. Wang, L. Oreopoulos, S. E. Platnick, S. von Hippel, K. Meyer, S. Light, and E. Wilcox, “Global reduction in ship-tracks from sulfur regulations for shipping fuel,” Sci. Adv., vol. 8, no. 29, p. eabn7988, 2022, doi:10.1126/sciadv.abn7988.
[20] V. Eyring, I. S. A. Isaksen, T. Berntsen, W. Collins, J. Corbett, O. Endresen, R. Grainger, J. Moldanová, H. Schlager, and D. Stevenson, “Transport impacts on atmosphere and climate: Shipping,” Atmos. Environ., vol. 44, no. 37, pp. 4735–4771, 2010, doi:10.1016/j.atmosenv.2009.04.059
Footnotes
[1] Somewhat over a quarter of anthropogenic CO2 enters the ocean, mean CO2 lifetimes are order of 500 years.
[2] Battery costs have fallen so far that this idea isn’t as absurd as when we first did these calculations in 2021. But even today the cost of the batteries for an electric cargo ship would be greater than the lifetime cost of fuel for a diesel cargo ship, but the battery-powered vessel would be able to carry far less useful cargo. That’s doubly damning when you consider that these ships—modern marvels that they are—are treated by operators as almost disposable. Individual cargo values can exceed the hull value, which means the economics of the shipping business favor cheap fast ships with surprisingly short lifetimes before they end up scrapped on a beach in Bangladesh.
“Electric Container Ships are a Hard Sail“
Dissenting view from Austin Vernon
[3] We choose 100 years as a convenient cutoff to approximate the “100-year global warming potential” (GWP100). Note that the real GWP100 of this carbon would be reduced by more than 5% due to the delayed release of the rest of the pulse.
[4] These results might beg the question: if downwelling to 100-200m results in very long duration storage, can’t the ship just have a long ‘inverse snorkel’ that pumps exhaust below that depth? Probably not. Between the fluid drag and the need for 10-20x compression of the exhaust gas at those depths, the vessel’s fuel requirement would rise drastically.
[5] A tremendous can of worms that’s thoroughly discussed elsewhere. Until recently, aerosol-cloud interactions from ship sulfur might have offset about 2-10% of anthropogenic warming:
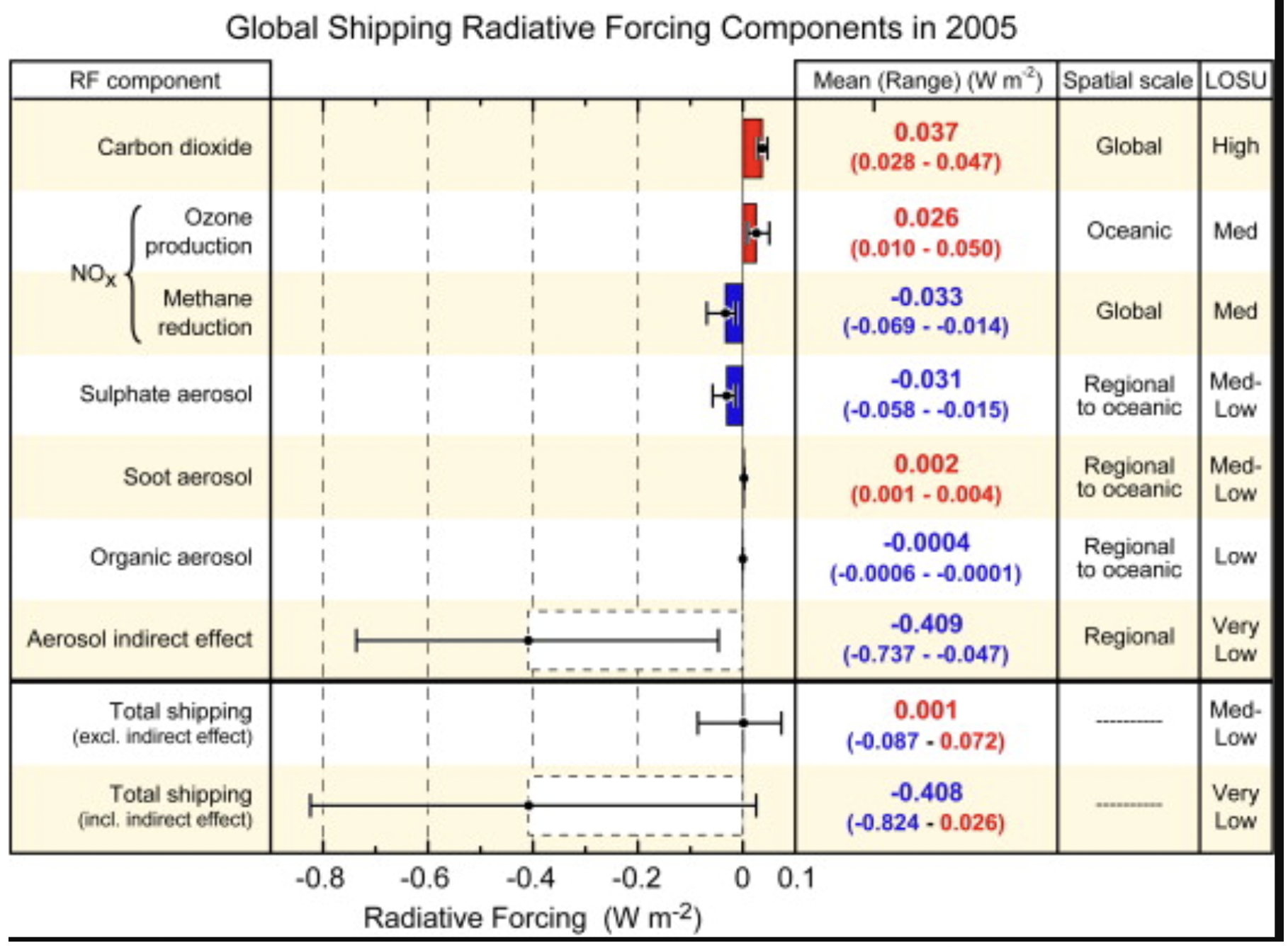
So it seems directionally clear (though with large error bars on just how much) that the IMO regulations will contribute to warming.
That might suggest that a marine diesel resulfurization campaign might be an effective way to offset 2- 10% of anthropogenic warming at zero or even negative cost. By modulating existing onboard scrubbers, resulfurization could even be tactical—release more sulfur in response to local humidity to maximize radiative and cloud-forming effects, and only far from shore to minimize possible health effects. That would seem like an extension of the logic behind projects like contrails.org; controlling aviation contrails is about undoing ongoing harmful geoengineering, this would go step one step further by reinstating and optimizing a “business as usual” helpful geoengineering entitlement.
But our opinion is that sulfur simply isn’t a good low-altitude geoengineering material. If we’re going to open the geoengineering box, we might as well do it in a more deliberate and effective way with fewer side effects. Sulfur aerosols kill people—Partanen et al. estimate that the tradeoff worldwide might be 10-50k deaths per year for a mere 0.5W/m2 decrease in radiative forcing. Even if you only did it in the open ocean, you couldn’t rule out that you were part of this. There are better ways [Refs 17, 18, 19].
Fuel desulfurization of course also increases net carbon emissions from fuel by a few percent; essentially hydrogen is stripped from some hydrocarbons (releasing CO2) and used to attack sulfur moieties on other hydrocarbons, giving us the great big piles of sulfur in Canada and elsewhere that will fulfill world sulfuric acid demand for many years after net-zero.
[6] Using cost estimates for available emission reduction technologies (scrubbers, exhaust gas recirculation, selective catalytic reduction) as a guide, we assembled a preliminary estimated cost for routing the exhaust to the bow of the vessel. The main hardware components consist of piping and fittings, fans, pumps, and a control system with an estimated total installed cost of $815,000. Normalized to the engine power, that yields a cost of $42/kW which is comparable to the installation cost of an exhaust gas recirculation system. Though the flow rates may be well matched, one potential complication in utilizing engine exhaust in an air lubrication system is in getting the exhaust to the front of the ship. A combination of additional blower power and valuable belowdecks space for piping would be required to bring the exhaust to the bow of the vessel without putting too much backpressure on the engine. Most engines are able to handle ~3 kPa of backpressure without noticeable effects on performance. Above that, each additional 3 kPa of backpressure reduces performance by roughly 1%. Simply installing a longer exhaust pipe (assumed to be 0.4 m in diameter) that extends from the engine to the bow of the vessel and pumps water to 1 m below the surface would create a back pressure of roughly 17 kPa, decreasing system performance by up to 6%. This means that we won’t be able to do without the high flow rate blower that is currently used in air lubrication systems. In fact, we will likely need a more expensive compressor that is built to withstand exhaust temperatures.
[7] Some ideas that would make that list more fun to read:
1) Metallic iron as fuel, eighty cents a kilogram, energy density comparable to coal when burned in air, electrochemical or thermophotovoltaic energy conversion. Either kept as oxides and recycled once ashore or dropped in the ocean for alkalization and fertilization purposes, leaving green belts of CO2-gobbling algae across the watery part of the world (which you might even get paid for).
2) A nuclear tugboat that always lives at least 200 miles offshore in international waters. Outside regulatory reach. Maybe it’s humongous and can pull ten supertankers at once.
3) Dry-ice depth charges. 10% of exhaust CO2 is freezable via counterflow heat exchange on an LNG vessel (freeze the CO2 out of the exhaust by cooling it using the -260ºF LNG input to the engine). Then drop the frozen CO2 overboard. Solid CO2 will sink in water and pool as a liquid on the seafloor; the storage duration in the mid-ocean will be millennia.
4) Giant airships riding scaling relations and well-mapped winds to victory, as discussed extensively elsewhere. Energy cost in J/(ton km) would be closer to ships than planes, but delivery time closer to planes than ships (a 500 ton airship cargo moving at 100kph would have the energy consumption of a plane a 10th of the mass…)
5) Cargo submarines, as confidently predicted by Arthur C. Clarke in Hazards of Prophecy:
“The submarine is a much more efficient vehicle than the surface ship, which wastes much of its energy on the production of waves. With the advent of nuclear energy, the high-speed, long range submarine envisaged years ago by Jules Verne is at last practical, but so far has been developed only for military purposes”
Clarke is right. Underwater travel is so much more efficient than surface travel that swimming as a sport had to institute a surfacing requirement after Masaru Furukawa dominated the 200 breaststroke at the 1956 Olympics by just staying submerged the whole time. Proposals to replace surface freighters with giant cargo submarines or towed underwater cargo pods have been floated a few times, often with the additional advantage that they’ll be able to go under Arctic sea ice to e.g. export stranded north slope gas to European markets.
[8] Climate change is full of these status quo bias breakdowns. Consider geoengineering. We already seed half the clouds in the northern hemisphere with soot aerosols, we already host basically artificial organisms (livestock or crop monocultures) on half the habitable land of planet earth, 95% of all mammals are us and our pets, we already corral the majority of terrestrial freshwater, a fifth of north American forests are managed tree monocultures…. And yet an experiment to launch a few hundred grams of sulfur in the sky is unacceptable meddling with the earth system. Wow…
[9] You might challenge this on the basis that wet sulfur scrubbers already inject an acid solution directly into the ocean, which is about the same degree of redirected-harm you’d occasion with the injected exhaust.
