There has to be a better way to make titanium
Titanium is an amazing material. How can we make it cheap and abundant?

On paper, titanium should be the world's #1 structural metal. There isn’t another metal with such great overall properties– incredible toughness and specific strength, light weight, exceptional corrosion resistance, and performance even at extreme temperatures. Titanium is also abundant, the 9th most common element in the earth's crust—cheap enough as ore to use in paint pigment and plastic filler[1]. And making titanium metal should take only half as much energy as making aluminum[2]. It should be cheap and ubiquitous.
Titanium was born for war—and in particular, to make war machines go as fast as possible. Its first major use was as the main structural material for the world's fastest planes (A-12 and SR-71) and submarines (Papa class).
But in reality, titanium is a rounding error in the world metals market – produced 5000x less than iron and almost 200x less than aluminum. Half a century after the US and Soviet governments conjured a commercial titanium industry from scratch to feed their cold war machines, we’ve seen virtually no progress in making the metal cheap or abundant. Learning curves that manifested quickly for aluminum and stainless steel simply didn’t appear for Ti— its “idiot index”, the cost of Ti parts as a multiple of the cost of ore and embodied energy, remains >10x that of steel. At $25-$50/kg, titanium is just too expensive to be widely used.
The total lack of progress keeps titanium in a reverse-Goldilocks zone where it loses to steel and aluminum on cost, and to composites on weight[3], and so is used only where its lightness and toughness are absolutely essential. Outside of aerospace, defense, corrosion-resistant process equipment, artificial joints and premium sporting goods, titanium is practically irrelevant.
Why is titanium so expensive?
Here’s how we get Ti today:
- We mine TiO2 as ilmenite ore, which is widespread and fairly concentrated at 40-65% TiO2. (~$1/kg Ti)
- The ilmenite is purified to synthetic rutile (90-95% TiO2) (~$2/kg Ti) then carbo-chlorinated to TiCl4 which can be distilled for very high purities. ($3-4/kg Ti)
- Next comes the main act: the Kroll process, reducing TiCl4 with magnesium metal TiCl4+2Mg -> 2MgCl2 + Ti. This is a touchy operation in which TiCl4 is meticulously metered into molten magnesium – too fast and the heat liberated in the reaction would vaporize the Mg metal. Since we have to remove the Mg and MgCl as gases, and the process happens well under Ti’s melting temperature, the metal we get initially is in porous and hard-to-use “sponge” form --full of bubbles like lava rock (~$6-8/kg Ti).
- The porous sponge is crushed, ground, mixed with alloying metals (useful Ti most often shows up as Ti90Al6V4), pressed, welded into 10-15 ton “consumable electrodes”, and homogenized in a vacuum arc remelter (VAR). (~$15/kg Ti)
- The ingots are then whittled down to saleable parts with sundry processes and techniques, which themselves are painstaking for many of the same reasons that Ti is so awesome as a structural metal – it’s hard enough to destroy tooling, forms a thick oxide film in O2, melts only at a staggering 1670ºC etc. … (~$25-60/kg Ti)
The cost of titanium roughly doubles with each of these steps[4] – and there are lots of steps!
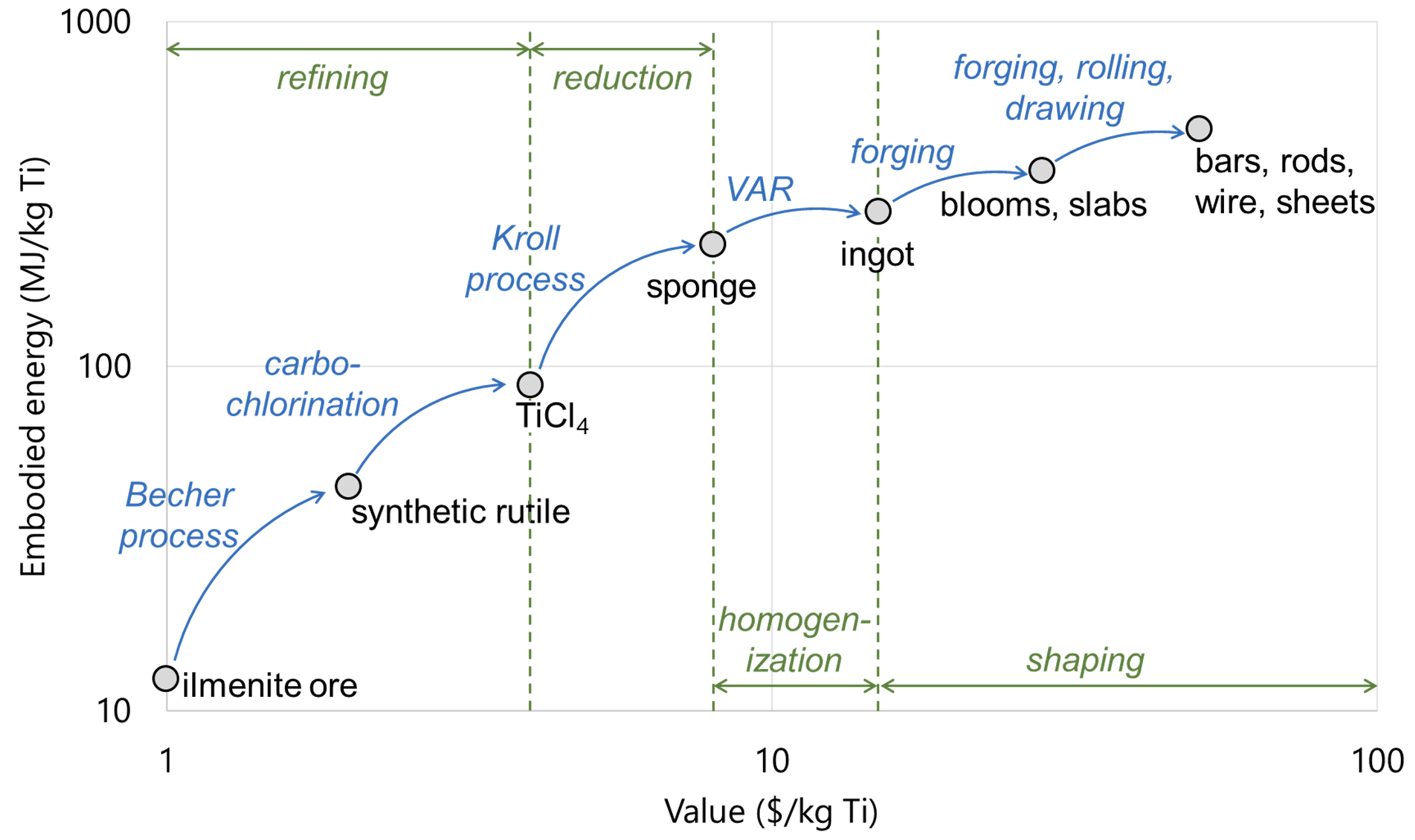
Cost and embodied energy for titanium mill products and intermediaries. In the refining stage Ti ore is cleaned of metallic (Fe, Al, Mg, Ca, V etc.) and semi-metallic (Si) impurities. The reduction stage provides the 4 electrons required to produce titanium metal from TiCl4. The homogenization removes porosity left over from the reduction stage to reach full-strength Ti. The shaping stage increases the surface area to volume ratio of the ingot into shapes which can be machined into useful parts.
Why are there so many steps here? Part of it is that titanium is both electropositive and fairly refractory—you need the uncomfortable combination of very high temperatures and a very reducing environment to get it into a useful form. But the bigger reason is that titanium is reactive—ferociously so. Molten Ti dissolves nearly every other metal. It readily forms oxides, nitrides, hydrides, and carbides. Titanium powders will ignite in carbon dioxide, and even in helium with as little as 9% oxygen. Sometimes this reactivity can be annoying, requiring an inert environment, or causing embrittlement or loss of material. Other times it can be deadly[5].
(But wait – isn’t titanium meant to be corrosion resistant? Shouldn’t it behave more like a staid transition metal than an electron-shooter like sodium? Like aluminum, titanium forms a passivating oxide on its surface that imparts its famous corrosion resistance, until about 600ºC. At that point, the titanium below the oxide skin begins to draw in oxygen at a meaningful rate, embrittling the part and exposing it to attack from whatever’s around.)
Titanium’s reactivity is especially punishing when you try to process it into useful shapes, which requires high temperatures where oxygen and anything else around gets in and ruins it. Consider for example just the ingot breakdown step. In order to make the ingots more pliable, producers heat them up to high temperatures and forge them in open air. As the surface area of the ingot increases, so does oxygen pickup, forming a brittle hide you have to shave off. That’s up to 50% of the metal you made, discarded useless on the mill room floor—just to forge an ingot! Frictions like this add up at every step on the value chain. Since Ti is only transformed incrementally at each step in this process, it all adds up to significant capital, time, labor and material loss tied into each step.
It's not a matter of optimization or learning rates
As bad as things seem, there’s good reason to believe that the way we make titanium today is pretty close to its cost floor.
The TiCl4 precursor to Kroll is a high-volume commodity chemical (>7 MMT/yr) that costs ~$4/kg Ti. Making TiCl4 is a mature process that’s substantially optimized already. Kroll also requires magnesium metal which is recycled on-site for ~$1.2/kg Ti[6] -- only a small multiple of the energy costs involved. Throw in a 15% profit margin, and you have arrived at the VAT excluded price for the cheapest Ti sponge quoted on the Shanghai Metal Market: ~$6/kg. So Kroll costs hardly more than its material inputs. 60 years of Ti production have whittled most of the inefficiencies away. The real cost and complexity comes later, in trying to work Ti sponge into useful parts.
Clearly, what titanium needs is reimagining of the whole process that reduces the number of steps in the value-add chain. Since cost roughly doubles with each step, eliminating one or two steps could easily save more than improving each one by 10%.
This fact is widely recognized—and has been for half a century. As Brian Potter writes in his excellent history of the Ti industry, even as the Kroll process was first scaling in the 1950s, newer and better ways of making titanium were widely expected to materialize. William Kroll himself expected that an electrolytic process similar to Hall-Heroult for aluminum would come to replace his method.
But the sad truth is that there have been many serious and well-funded attempts to reinvent Ti production over the last 50 years, and all have failed to meaningfully displace the Kroll-centric approach we use now. Let’s look through what has been tried, and see what we can learn:
The cruel lessons from 60 years of failing to reinvent titanium
People have tried all sorts of methods: electrolysis, plasma, alternative reductants, additive manufacturing. None ever produced full-density Ti cheaper than Kroll:
1960s – Dow-Howmet/TIMET/RMI electrolysis – Electrolysis of TiCl4 to porous Ti.
1994 – Hydrogen plasma reduction – Thermal plasma for reduction of TiCl4 to Ti powder.
2000 – Armstrong process – Na reduction of TiCl4 to Ti powder.
2000 – Farthing-Fray-Chen (FFC) Cambridge & Ono-Suzuki – Electrolysis of TiO2 to produce porous Ti.
2003 – Cardarelli-Rio Tinto electrolysis – Direct electrolysis of molten TiO2 to molten Ti.
2003 – Ginatta electrolysis – Electrolysis of TiCl4 in fluoride/chloride salt to make molten Ti.
2004 – MER Corp electrolysis – Electrolysis of Ti oxycarbide to porous Ti.
2012 – ADMA process – Hybrid H2/Mg reduction of TiCl4 to TiH2 powder.
2013 – Hydrogen Assisted Magnesiothermic Reduction (HAMR) – Hybrid H2/Mg reduction of TiO2 to Ti powder.
We take away four main lessons:
- Start with TiCl4. Trying to start from TiO2 makes things much harder, and can’t meaningfully reduce costs.
- Any new process will have to make dense Ti directly if it’s going to be useful. Kroll already makes porous Ti efficiently. The problem is that porous Ti simply isn’t a valuable product.
- Beware additive techniques. These are the most promising of the alternative approaches, but signs indicate that they won’t reach the scales we’d need to revolutionize Ti.
- Beware electrolysis. It’s the most frequently attempted and least successful of the alternative approaches. Ti has several cruel chemical features that make it especially challenging to manipulate in solution.
1) Trying to start with TiO2 makes things much harder
Most of the attempts reinvent Ti since the early 2000s have tried to substitute TiO2 for Kroll’s TiCl4 starting material. That's a way to save a few bucks on feedstock costs-- and the only possible route to getting Ti as cheap as Al or Mg. But unfortunately, today none of those projects produce titanium at commercially relevant tonnages. We think that much of the problem has to do with the hassle of purifying TiO2.
The vast majority of titanium used on earth is sold not as Ti metal, but as white TiO2 pigment. TiO2’s whiteness depends strongly on its purity. So the TiO2 pigment industry has come up with an excellent way of making it as pure as possible: carbo-chlorinating synthetic rutile, fractionally distilling the various volatile metal chlorides until just the TiCl4 remains, and then burning the purified TiCl4 in oxygen to get back to pure TiO2.
As complicated as this sounds, the chloride distillation route is just about as good a way as is thermodynamically possible for purifying a metal as chemically “sticky” as Ti. Since Ti metal has to meet strict purity standards (~0.1wt% for most elements), trying to make metal directly from TiO2 saddles you with a serious purity problem right at the start.
TiCl4 also serves a valuable function in keeping oxygen out of your reaction mixture. Oxygen is fiendishly soluble in Ti metal—so much so that its presence makes metallothermic reduction almost impossible.
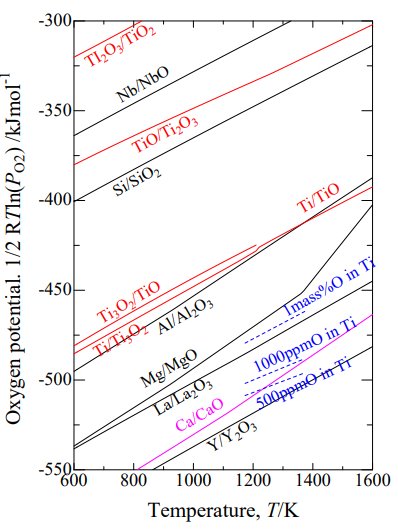
The thermodynamic nightmare of trying to separate Ti from O in a reaction mixture. Commercially pure titanium requires <1000 ppm O which is accessible only by TiO2 reduction with calcium and yttrium (the lines for magnesium and lanthanum appear to intersect that of Ti-1000 ppm O at a low enough temperature, but in practice those reactions are starved for activation energy and bogged down by solid-state diffusion)
Just say no to TiO2. TiO2 that is pure enough to be a feedstock for Ti metal is more expensive than TiCl4 anyway, and makes the reduction of Ti vastly harder. We want to delete steps, but not at the cost of pure titanium.
2) Porous Ti is not a valuable product
Most of the inefficiencies in the Kroll process have been whittled away over the last 60 years. At this point, Ti sponge sells at a price that is very nearly the sum of the cost of TiCl4, a high-volume commodity chemical, and the cost of producing magnesium with a well tamed electrolytic process. What exactly would you improve on?
Imagine you did invent a magical new process for making porous Ti sponge. Say it’s half the capital cost of Kroll and a quarter of the energy cost—just above the thermodynamic minimum for making Ti. You buy the cheapest pure feedstock that gets you the purity you need, TiCl4 at ~$4/kg. If you want a 10% profit margin, that leaves you only ~$2.3/kg Ti to finance, operate, and maintain your process if you’re going to compete with ~$7/kg Kroll sponge. Kick in $1.35/kg Ti for capex (half of Kroll’s capex of $30/kg Ti per year amortized at 8% for 20 years)[7], $0.5/kg Ti for electricity costs, and ~$0.5/kg Ti for labor and maintenance—and even though your process is magical, it’ll still be borderline unfinanceable if all you can sell is Kroll-equivalent sponge!
Titanium sponge just isn’t where the value is. Saving a few tens of cents on $7/kg Ti that will later be turned into mill products that cost as much as $50/kg Ti won’t move the needle.
3) Additive manufacturing is unlikely to give titanium the volume it needs
So if sponge isn’t where the value is, maybe we need an all-new way to turn sponge Ti into finished parts?
A lot of people have seen it this way in recent years, and so there’s been a lot of excitement about additive manufacturing for Ti parts. It makes sense in some ways. After all, subtractive manufacturing sucks for titanium – its hardness and low thermal conductivity chew through tooling[8], and its reactivity costs you material in high temp processing. Both Ti and additive manufacturing are mostly used at the top of the market where expense hardly matters. And a lot of the newfangled ways you might make Ti give you spongy or powdered metal that needs additive post processing to get to full density anyway.
But while additive manufacturing with Ti powder does some specific things very well, one thing it won’t do is make Ti cheaper than it is today. Additive manufacturing tools are generally less capital intensive but have lower throughput, while conventional methods get economies of vertical scale. This has played out in the market for a few years now and cost analyses show that there’s a breakeven manufacturing volume above which conventional Ti production is more cost-effective. The exact value varies but this paper suggests that conventional Ti casting beats additive manufacturing at scales above a few hundred parts (valve bodies made with hot isostatic pressing vs. conventional forming), and this analysis of a landing gear reports that high pressure die casting is more cost effective than selective laser sintering above ~40 parts produced.
There are also lingering concerns that the main approaches to additive Ti leave micro-pores in the final metal product that concentrate stress and make weaker overall parts, especially under cyclic loading. Fear of microporosity pushes additive manufacturing to use high-cost ($70-200/kg) ultra-spherical powders in order to minimize pore volumes[9]. But concerns over part strength have persisted especially among the usual sectors for early adoption—defense, aerospace, and medical equipment– where failures can be catastrophic and the cost for Ti is borne easily.
Additive manufacturing does shine for low production volumes of highly complicated parts that conventional manufacturing struggles to make at all (see SpaceX’s SuperDraco). But an abundant Ti future needs a new method that can make Ti at high volumes and low cost and full strength, and that method probably won’t be additive manufacturing.
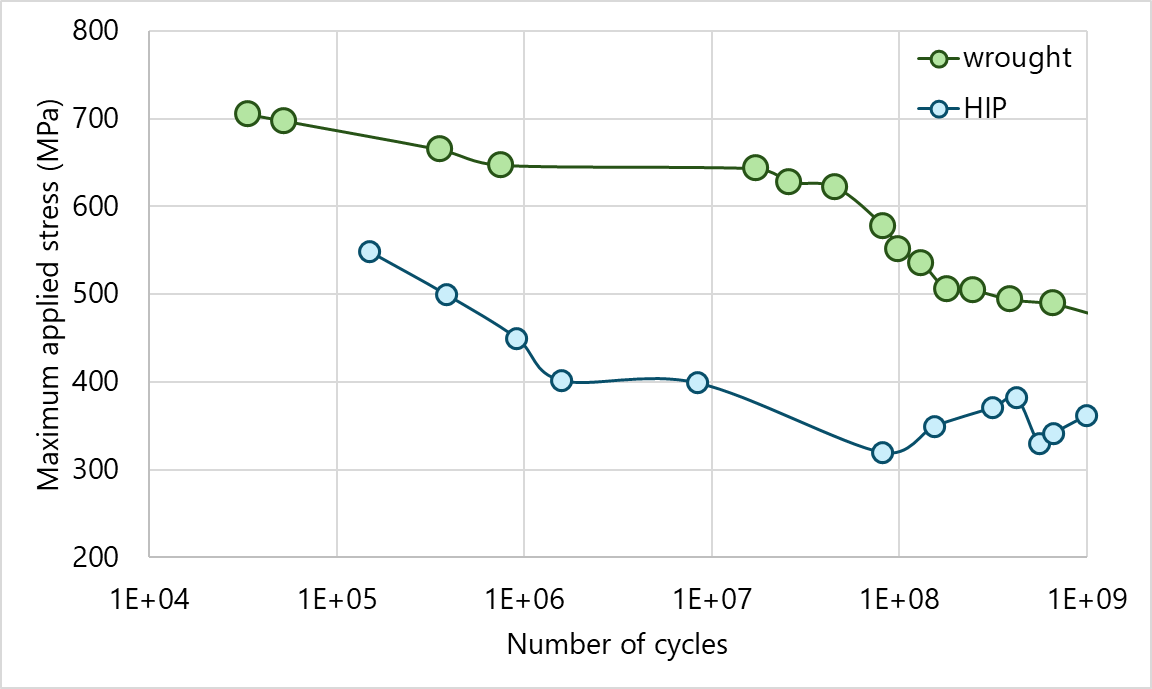
There are some long-lasting concerns about the durability of additively manufactured Ti parts, which can contain thousands of stress-concentrating voids per cc of material. This graph shows applied stress vs. number of cycles for parts made of wrought Ti-64 alloy versus for hot isostatically pressed parts made with Ti-64 powder. Adapted from Cheng et al. (2022) and Rao and Stanford (2021).
4) Electrolysis isn’t out of the question but it comes with severe challenges
Electrolysis seems like a logical approach to making titanium. After all, the development of an electrolytic process for aluminum took it from a precious metal used only in jewelry to a ubiquitous structural material in just a few decades. Could it unlock similar cost-savings for titanium?
Probably not, we think:
Ti has too many valence states in solution. Titanium, unlike aluminum, adopts multiple valence states which engage in a phenomenon called redox cycling in an electrolyzer. Ti2+ can flow across the cell in one direction, drop off an electron, and flow backwards as Ti3+. So current flows through the cell for no reason, generating no titanium metal and producing nothing but joule heat! Electrolytic magnesium produced for the Kroll process at ~30% voltage efficiency and >90% current efficiency is just a more efficient way of packing electrons into a titanium feedstock.
Ti melts at too high a temperature, but lowering the temperature makes things worse. Part of the magic of the Hall-Heroult process for aluminum is cryolite, which lowers the melting temperature of Al2O3. Since Al metal also has a relatively low melting temperature (660ºC), a Hall-Heroult cell can operate at reasonable temperatures and still pour molten aluminum into shapes that can be sold directly to customers.
If only that were possible for a Ti electrolyzer, it could delete the costly post-processing steps in making Ti parts, and make finding compatible electrolyte and electrolyzer materials that much easier. But Ti’s 1670ºC melting point makes that impossible.
You could make building your electrolyzer much easier by running it below titanium’s melting point. Some people have tried this. But at meaningful current densities, the product will then be dendritic titanium – tree-like metal crystals embedded in frozen electrolyte. What would it take to make this titanium useful? First, you must clean the titanium of the salt and then melt it down to make homogenous titanium stock – the same steps that make Kroll’s sponge titanium expensive. A non-starter.
Overall, electrolysis is the most-frequently attempted and least successful of the alternative approaches to Ti. People will keep trying—but we will be surprised if anyone gets it to work.
Tempering our expectations
People who love titanium tend to compare it to steel and aluminum since Ti’s base properties are so much better. They’ll also point out that the ore is cheap and abundant and that the metal costs vastly more than it should, a staggering 60x over its theoretical cost floor (the cost of ore plus energy). That kind of argument makes it easy to convince yourself that Ti should be a main structural material if not the main structural material of the future—if only we could find a better way to make it.
But it’s worth offering a moderating opinion, both on how cheap titanium can really get, and on how useful it is.
Why you can’t say that titanium beats aluminum or steel categorically. Consider the bicycles used in the Tour de France, ultra-premium machines made of the best possible materials. From 1904 to 1994 the Tour de France was won on steel bikes. From 1995 to 1998 it was won on aluminum bikes. And then from 1999 until now it’s been won exclusively on carbon fiber bikes. Why was Ti passed over completely?
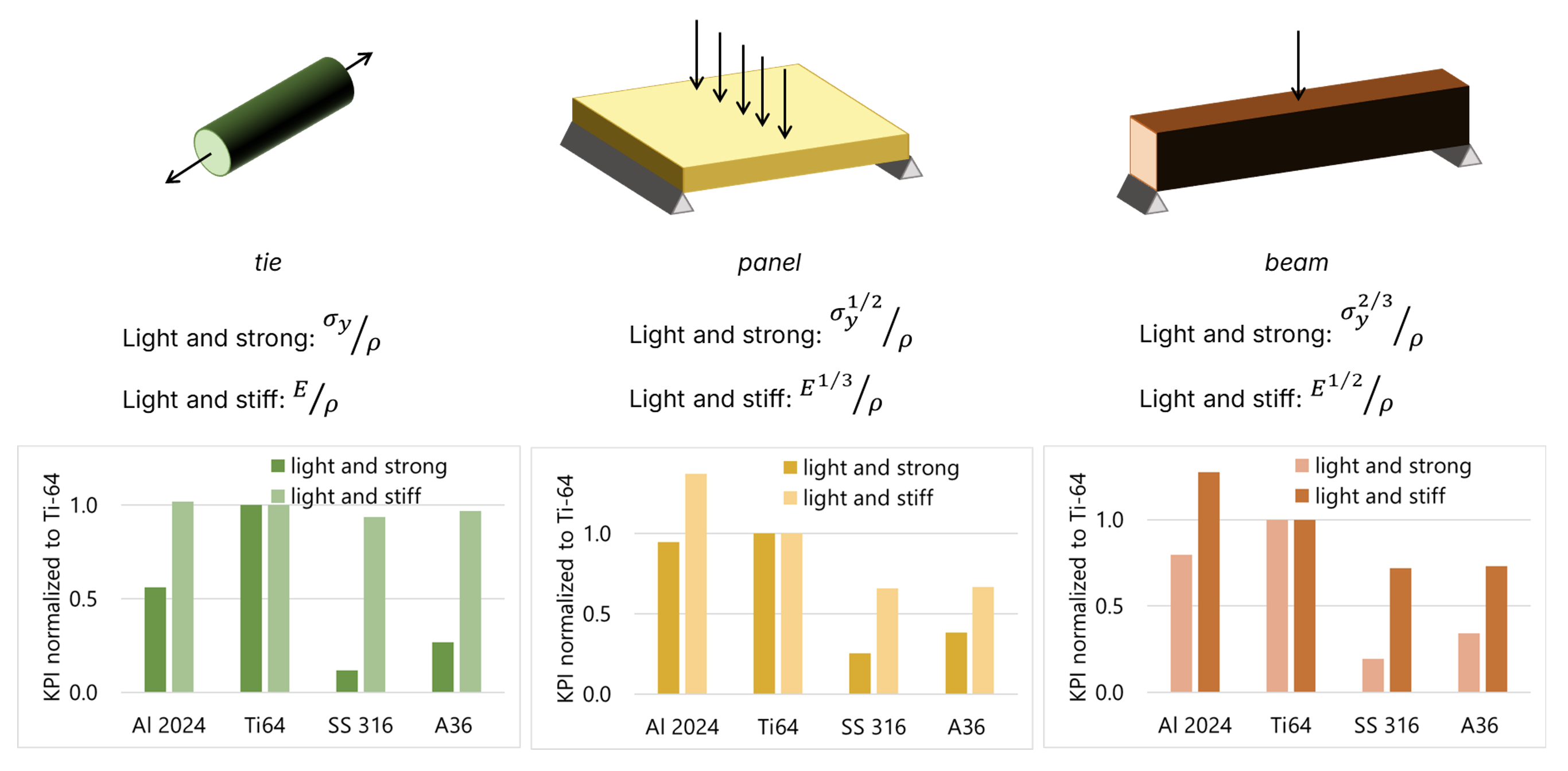
Titanium’s huge advantages in specific strength over aluminum and steel are muted when you look at the structural metrics that actually matter. The bar plots compare these metrics for an aluminum alloy (Al 2024), a titanium alloy (Ti-64), stainless steel (SS 316), and a mild steel (A36), all metrics normalized to Ti-64. For cost parity in a tie (tension member) titanium ties need to be <2X the cost of aluminum, <10X the cost of stainless steel, and <4X the cost of mild steel. The multipliers are even lower for panels and beams.
Strength-to-weight ratio isn’t everything. Larger-diameter tubes in Al bike frames are stiffer than Ti and can even be stronger—not based on material properties, but based on geometry. In a bending tube in a bike frame, the critical buckling load scales with the cube of the outer diameter, but only linearly with stiffness. If you want a stiff and strong frame, or one that’s easy to shape into aerodynamic forms, aluminum with its lower density and better formability can end up as the winning choice.
This type of dynamic repeats all across industry. Properties like density end up being just as important as specific strength, and lesser metals that are easier to form into the right geometry end up competing with and even beating titanium.
Why titanium will always be pricier than Al or steel. Hopefully we’ve landed this point by now: Ti is extremely hard to work with. The reasons why are written in its atomic structure. It’s semi-refractory and a sponge for nearly everything around it. The purity of the feedstock and reducing agent, inertness of the reactor volumes, and metal handling at elevated temperatures are crucial aspects that will increase capital and labor costs and decrease material utilization, no matter how clever your process is. Unlike titanium, aluminum and steel smelters operate in atmospheric conditions, the molten metals can be poured in open air to form shapes that brokers can store on shelves, and they are both compatible with common high temperature materials like alumina, silica, and graphite[10]. None of that works for titanium.
Where does this all leave us?
Back when it was young and promising, ARPA-E ran a program called METALS which sought to reinvent many of the light metals for the modern era. They estimated substitutional elasticities for different structural metals to come up with some cost targets, how cheap these metals would have to get to really change the world. For Ti it was $10/kg at the ingot level. That’s the cost where they thought Ti could start to meaningfully displace steel and aluminum parts.
Is that possible? Despite all the harsh lessons over the last few pages, we think so.
All this reflection on past failed attempts to reinvent titanium production makes us think that the straightest line to cheaper titanium is to delete steps wherever possible and to not shy away from energy intensity. Paying extra energy for purification and reduction could get you to fewer total steps and direct castability. Fewer steps is key since titanium is a refractory metal that can soak in nearly everything around it, leaving it worse off. Every step wastes material and makes it harder to meet purity standards.
At this intermediate stage in our brainstorm on the topic, what looks best to us is a single-step metallothermic reduction of titanium chloride that produces molten titanium directly. That’s like a more energy-intense version of Kroll. With 150% of Kroll’s capex and opex you could make ingots that hit the ARPA-E target. Better yet, using more energy in the reduction step could leave you with molten Ti so you could skip the ingot step entirely, getting you customer-ready bars and sheets for less than ingot prices. If somebody could do that, we think they could beat Kroll Ti by wide margins.
It’d be hugely energy intensive—but that could be OK. People are already making metals off-grid with dirt cheap electricity. We’ve said before why we shouldn’t be shy of energy intensity in materials production— falling energy costs have always been a main driver for lower material costs, and energy-intensity has always gone hand in hand with building the world out of much cooler materials [11].
High energy intensity, direct castability, deleting steps. If you’re also thinking about titanium along similar lines, please get in touch.
Footnotes
[1] Granted that titanium is 10 times rarer than iron, but it is 6, 55, and 67 times more abundant than manganese, chromium, and nickel respectively – the high-percentage additives that make iron last long enough to be useful
[2] The free energy of formation of aluminum oxide and titanium tetrachloride, the main feedstocks that feed the aluminum and titanium reduction technologies, are ~180 kcal/mol metal.
[3] Ti complements composites better than aluminum. Aircraft are optimized for lighter weight, so the newer 787s have more composites like carbon fiber and fiberglass reinforced polymers than the older 747s. Aluminum corrodes when in contact with carbon fiber, so these junctions are made of titanium instead.
[4] The cost of ilmenite ore containing 50% TiO2 is ~$0.3/kg and synthetic rutile containing 95% titanium dioxide is ~$1.2/kg. The cost of titanium sponge was derived from the average US import price as reported by USGS minus 15% ad. valorem and 20% profit. The costs of titanium mill products (ingots to wire) are derived from the average US export price reported by USGS.
[5] John D. Clark recounts in Ignition! why it’s so dangerous to use titanium in rocket components:
There was a good deal of interest in titanium at that time, as many rocket engineers wanted to use it…. On December 29, 1953, a technician at Edwards Air Force Base was examining a set of titanium samples immersed in RFNA [red fuming nitric acid], when, absolutely without warning, one or more of them detonated, smashing him up, spraying him with acid and flying glass, and filling the room with NO₂. The technician, probably fortunately for him, died of asphyxiation without regaining consciousness. … Initial intergranular corrosion had produced a fine black powder of (mainly) metallic titanium. And this, when wet with nitric acid, was as sensitive as nitroglycerine or mercury fulminate. (The driving reaction, of course, was the formation of TiO₂.) Not all titanium alloys behaved this way, but enough did to keep the metal in the doghouse for years, as far as the propellant people were concerned.
(In fairness this story also highlights the murderous oxidizing power of red fuming nitric acid, RFNA, which is used to make aqua regia capable of dissolving noble metals like platinum and gold.)
[6] The Kroll process uses ~1.3 kg Mg/kg Ti. The capital cost required to build a magnesium electrolyzer is 3.3 $/kg Mg per year and electricity consumption is ~13 kWh/kg Mg. At 8% discount rate over a 20-year lifetime, and electricity cost of $70/MWh, the cost of producing magnesium is ~$1.2/kg Ti.
[7] In 2006, Allegheny Technologies announced the approval of a $325 million sponge facility capable of producing 24 million pounds (~30 $/kg Ti per year) in Rowley, Utah. At 8% discount over a 20-year lifetime, the capital cost on Kroll sponge is ~$3/kg Ti. In comparison, aluminum smelters are built for ~$5/kg Al per year.
[8] Titanium’s strength at high temperatures and low thermal conductivity localize heat at the tool edge and wear it down quickly. Its high chemical reactivity at cutting temperatures lead to galling and atomic migration from the tool to the workpiece (diffusion wear). Its relatively low elastic modulus causes the workpiece to chatter while being cut leading to low dimensional tolerance. All these issues culminate in parts that require higher stock to part ratio than steel or aluminum, high skilled labor, and special cutting tools.
[9] The true cost of production of the most popular grade of titanium powder (Ti-64) can be as low as $70/kg Ti according to Santiago-Herrera et al., and as high as $200/kg Ti as quoted by other suppliers.
[10] Molten titanium will react with all metal oxides except yttria, zirconia, and calcium oxide. Due to calcium’s volatility at titanium’s melting point, titanium can also react with calcium oxide to form calcium vapor, if there is no excess of the latter. Titanium will react with carbon to form titanium carbide at temperatures as low as 400ºC.
[11] A relevant sidebar here is that the discovery of alumina dissolution in cryolite was not the only enabler for aluminum as a commodity metal – cheap electricity from hydroelectric plants was just as instrumental.
